The biotech and pharma customers can use Aptar’s three-phase Activ-Polymer protective solutions to meet specific drug long-term and in-use stability requirements
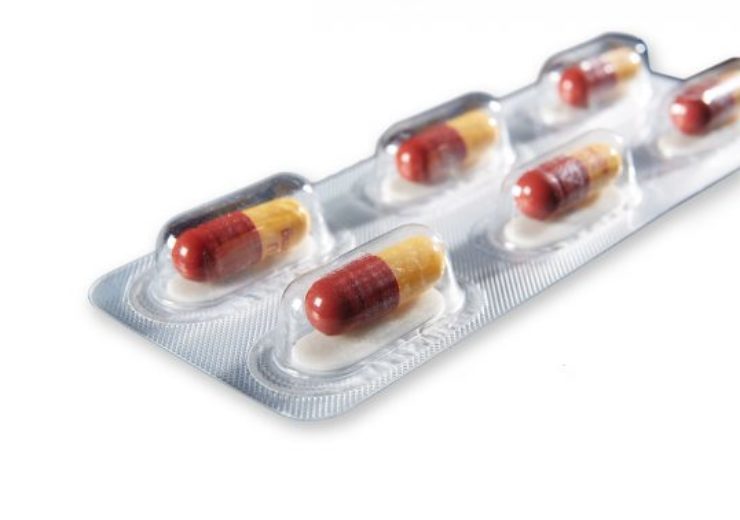
Image: The FDA has approved Aptar’s Activ-Blister packaging solution for oral solid dose HIV prevention medicine. Photo: courtesy of AptarGroup, Inc.
Drug delivery and packaging solutions provider Aptar has secured approval from the US Food and Drug Administration (FDA) for its Activ-Blister packaging solution for oral solid dose HIV prevention medicine.
The firm said that a major pharmaceutical company, which focuses more on the development of HIV treatment and prevention solutions, has developed the oral solid dose drug.
According to the packaging company, the latest move represents the first FDA approval of Aptar’s Activ-Blister packaging solution and an advanced application process developed by Aptar CSP Technologies.
The system offers protection to oral solid drug products with a 3-Phase Activ-Polymer solution, which is fully integrated into the blister package.
Activ-Polymer technology of Aptar will scavenge volatile organic compounds and emit aromas
The three-phase Activ-Polymer technology can be customised as per the formulation of drug developer and provides a broad spectrum of specific drug protection such as moisture adsorption and oxygen and odour scavenging.
Aptar’s technology also holds the capacity to scavenge volatile organic compounds (VOCs) and emit aromas.
The biotech and pharmaceutical customers can use Aptar’s portfolio of 3-Phase Activ-Polymer protective solutions to meet specific drug long-term and in-use stability requirements and maintain therapeutic efficacy in a patient-friendly packaging configuration.
Aptar president and CEO Stephan Tanda said: “I am very pleased to announce this recent FDA approval of our proprietary Activ-Blister technology.
“This is a significant step that further validates the expanding portfolio of solutions offered by Aptar CSP Technologies.
“We will continue to leverage our proprietary 3-Phase Activ-Polymer™ technology to help our customers with unique protective formulations that derisk their drug development process and help strengthen their own offerings. The ultimate result is that we are creating meaningful solutions that help improve and save lives.”
In September this year, AptarGrouphas has partnered with PureCycle Technologies to speed up the integration of Ultra-Pure Recycled Polypropylene (UPRP) into dispensing applications.
The advanced recycling process, patented by PureCycle, separates colour, odour and any other contaminants from plastic waste feedstock to transform it into UPRP resin with virgin-like properties.
