The Activ-Film material will allow the implantable medical device to control humidity and enhance the accuracy of readings
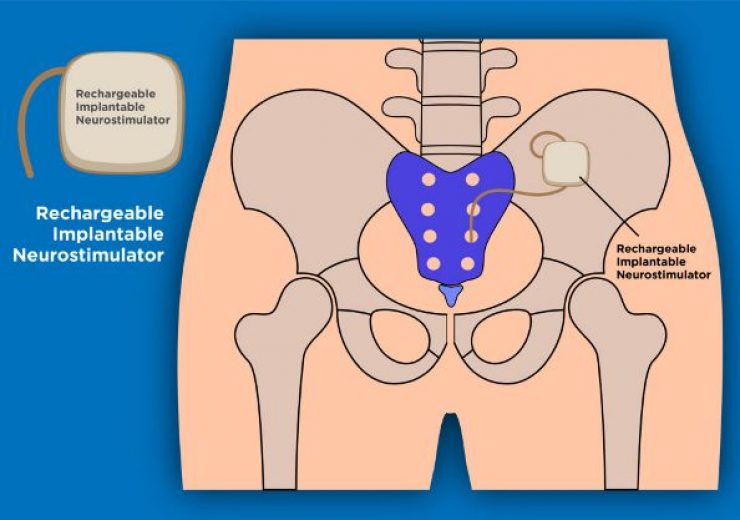
Aptar has secured FDA approval for Activ-Film technology for use with rechargeable implantable neurostimulator. (Credit: AptarGroup, Inc.)
Aptar CSP Technologies has secured approval from the US Food and Drug Administration (FDA) for its Activ-Film technology for use with rechargeable implantable neurostimulator (INS).
Designed to treat urinary and bowel dysfunction, the medical device uses the Activ-Film material to control humidity, enhance the accuracy of readings and expand use life.
The Activ-Film material is said to leverage Aptar CSP’s patented 3-Phase Activ-Polymer platform technology for the management of the internal atmosphere and adsorbing moisture in the device.
It will help avoid the accumulation and affecting the stability and performance of the implant.
The humidity control within the device will also help protect the battery, in addition to avoiding degradation during its use life.
Aptar CSP Technologies commercial operations vice president Badre Hammond said: “This is another example of our consistent commitment to helping customers bring advanced solutions to patients and improve user experiences and outcomes.
“We are dedicated to continued material science innovation that facilitates the development and commercialisation of next-generation implantable medical devices.”
Earlier this month, Aptar CSP said that it provided its Activ-Film technology to protect two new at-home Covid-19 tests.
The advanced film technology is also used in different forms for use in a range of applications such as oral solid dose drugs, drug delivery systems and probiotic, in addition to protecting various medical devices.
Aptar stated that the material science technology can also be customised to offer integrated active solutions, which adsorb moisture, scavenge oxygen, odour, or VOCs, emit aromas, or minimise pathogens.
