Under the collaboration, Stevanato Group will offer and produce its glass pre-fillable syringe Alba assembled with the Integrated Spray Module (ISM) of Recipharm’s soft mist inhaler technology, the Pre-Filled Syringe Inhaler (PFSI)
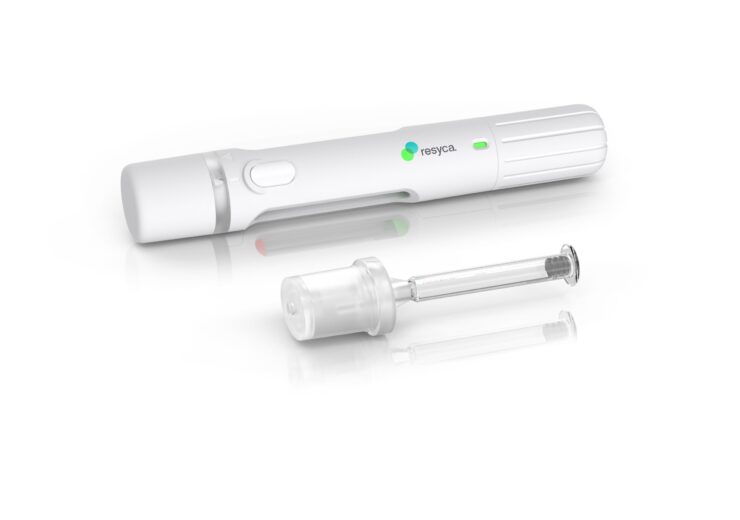
The pre-fillable syringes are used in soft mist inhalers for the inhalation of biological products. (Credit: Business Wire/Stevanato Group S.p.A.)
Stevanato Group and contract development and manufacturing organisation (CDMO) Recipharm have partnered to develop and produce pre-fillable syringes for use in the latter’s soft mist inhalers.
Under the collaboration, Italy-based Stevanato Group will offer and produce its glass pre-fillable syringe Alba assembled with the Integrated Spray Module (ISM) of Recipharm’s soft mist inhaler technology, the Pre-Filled Syringe Inhaler (PFSI).
The partnership shows Stevanato’s strengths as a one-stop shop that provides support for contract drug manufacturing companies’ drug development programmes from the clinical stage to market release.
Stevanato said that its Alba syringes include an internal coating based on silicone oil that is cross-linked with the glass surface to minimise sub-visible particle release and ensure better performance during delivery.
The Alba syringe together with Recipharm’s soft mist inhaler technology will deliver sensitive medication items more effectively to the respiratory airways, offering .biopharma firms a containment solution with improved stability and safety.
Under the deal, Stevanato will assume responsibility for the overall primary drug packaging, while CDMO will focus on the design and production of the spray technology, the inhalation device, and the fill and finish of the product.
Stevanato Group CEO Franco Moro said: “Creating a network of strategic collaboration partners is an important element of our long-term strategy to match customers’ needs and address self-administration trends in patient care with user-friendly drug delivery devices that provide variable and accurate dosing.
“This agreement marks another key step in broadening our high-value solutions and integrated capabilities as we continue to diversify and enhance our presence in the drug delivery market of pen injectors, auto-injectors, inhalers, and wearable pods.”
Resyca, a joint venture between Recipharm and Medspray, developed, produced, and obtained the licence for Recipharm’s soft mist inhaler (PFSI), which is provided as a pre-filled and ready-to-use inhalation device.
