QuVa Pharma uses the new flag label design for syringe product portfolio of compounded sterile products
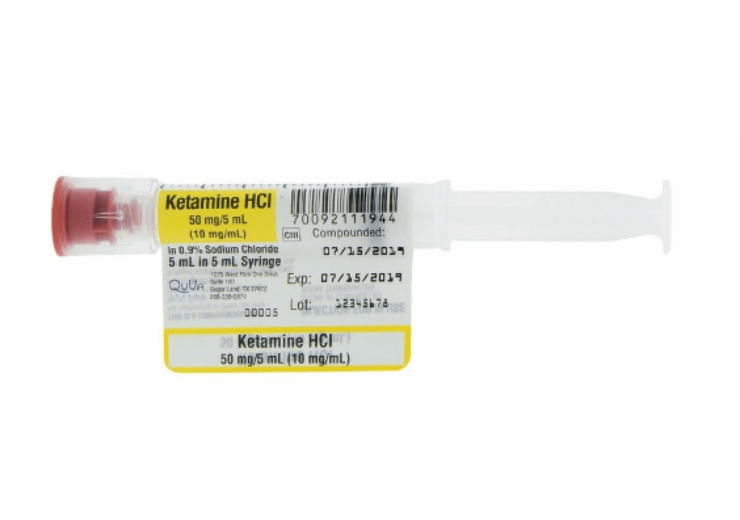
Image: QuVa Pharma has launched new syringe flag labels to improve patient safety. Photo: courtesy of PRNewswire / QuVa Pharma, Inc.
QuVa Pharma has unveiled new syringe flag labels to improve patient safety and reduce the risk of drug and dosing errors.
The company applies the new flag label design for its syringe product portfolio of compounded sterile products (CSPs). It has also filed a design patent application for new syringe flag labels.
The new labels have been designed in consultation with QuVa Pharma’s customers
QuVa Pharma has developed the new labels based on the feedback of the customers and started converting all syringes to the new enhanced label style.
QuVa Pharma, which is a provider of compounded sterile products, has facilities in Sugar Land and Temple of Texas, and Bloomsbury of New Jersey.
The company has licence to provide compounded preparations in all 50 states of the US. It supplies a range of sterile and ready-to-use compounded products, including product compounded from bulk drug substance to alleviate drug shortages.
It offers a portfolio of products across pain management, anti-infective, OR Syringes, labour and delivery therapeutic areas.
QuVa Pharma co-founder and CEO Stuart Hinchen said: “Improving patient care and safety by reducing medication errors is essential. Our commitment to achieving higher standards through partnering with our customers has led us to an innovative enhancement to the labels for our syringe portfolio.
“Delivering an enhanced syringe label with full drug and dosing visibility, regardless of syringe orientation, is an advancement in quality aimed at reducing drug and dosing errors. This is another example of our development track record that will help our hospital partners meet their clinical needs.”
Earlier this month, LOG Pharma Packaging has unveiled its patent-pending line of ActiveGuard Connect smart packaging solutions.
LOG Pharma, in collaboration with Water.IO, has developed ActiveGuard Connect to address the lack of patient adherence to prescription medicine or supplementation of nutraceuticals.
The new product is expected to facilitate indication of taken doses and tracks temperature within the bottle, and reminds or alerts patients when required to drive adherence.
