The new facility at the Rockford, Illinois site is expected to enhance the equipment and capacity for injectable biologics and other self-administered therapies
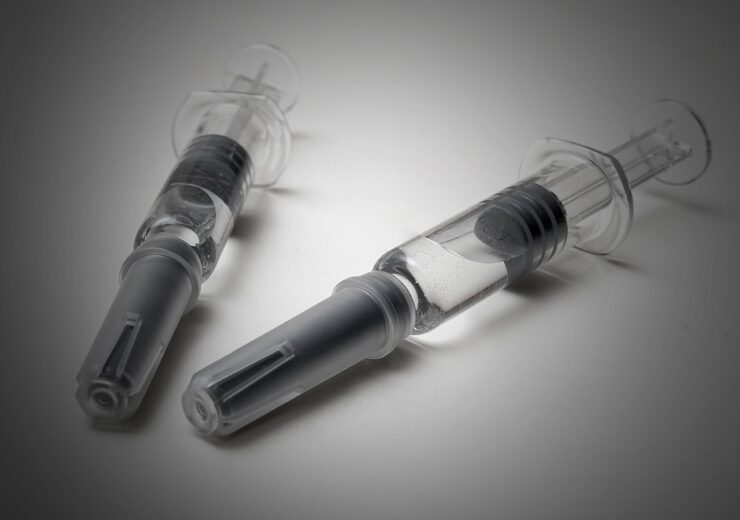
The facility will support packaging of vials, pre-filled syringes, auto-injectors, and pen-cartridge combinations. (Credit: PCI Pharma Services)
PCI Pharma Services, a contract development and manufacturing organisation (CDMO), has unveiled its plans to invest $50m to develop a new Centre of Excellence facility in Rockford, Illinois, US.
The new 200,000ft² facility is expected to enhance the capacity of the US-based firm in injectable drug-device combination product assembly of both biologics and small molecules.
According to the CDMO, the new site is the second expansion building after the success of its Philadelphia Biotech Centre of Excellence.
Expected to be operational in the summer of 2024, the facility will feature over 20 customer suites with multi-format machines for the assembly and packaging of vials, pre-filled syringes, auto-injectors, and pen-cartridge combinations.
PCI said that the machines can be utilised to make glucagon-like peptide 1 agonists (GLP-1) class of drugs prescribed to treat diabetes and obesity. They can also be used for oncology treatment and autoimmune diseases.
The company claimed that the Rockford location will house product testing capability, the latest top-load cartooning technology, and injector and pen assembly equipment.
Additionally, the site will have on-site cold storage, high-speed vial labelling, assembly, and packaging of multi-format autoinjectors, serialisation, testing, and drug product release.
PCI Pharma Services CEO Salim Haffar said: “We see the tremendous impact that we can make with patients with this investment.
“The need for injectable drug-delivery device combination product capacity and expertise is critical, and we are responding with a world-class facility to address the future demands of our global clients so they can focus on developing therapies to improve the lives of patients with serious chronic conditions.”
The investment is expected to boost the firm’s speciality drug presence in the Midwest region. It will also add clinical and commercial sterile fill-finish capabilities in Madison, Wisconsin.
The facility will act as a second hub within the US, expanding from its base in the Northeast, with Bedford, New Hampshire’s campus growth in aseptic processing, the CDMO added.
According to the firm, the expansion project is anticipated to generate 250 jobs in the region in the next two years, with scope for growth in the following years.
